Conventional development and evaluation of ocular drug products require in vitro and in vivo animal studies which can be expensive, time consuming and even ethically questionable. A computational software tool that can simulate ocular drug delivery and its interaction locally and systemically within the whole-body will be exceptionally beneficial. It can provide an accurate and efficient computational platform to virtually test, design, and develop ocular drug products as well as to facilitate translational applications from bench to bedside.
The majority of blindness and vision impairment in the US are age-related diseases, such as glaucoma and diabetic retinopathy, requiring drug intervention for disease management. The eye has many physical and biological barriers that limit a drug’s bioavailabilty for absorption and restrict systemic drug administration since drug levels required for treatment efficacy may be too toxic to the rest of the body. The complex anatomy and physiology of the eye require highly sophisticated physiologically-based pharmacokinetic (PBPK) model of the eye combined with biophysics-based ocular drug delivery model and whole body pharmacokinetic (PK) and pharmacodynamics (PD) to estimate the PK profile of a drug in the eye after administration.
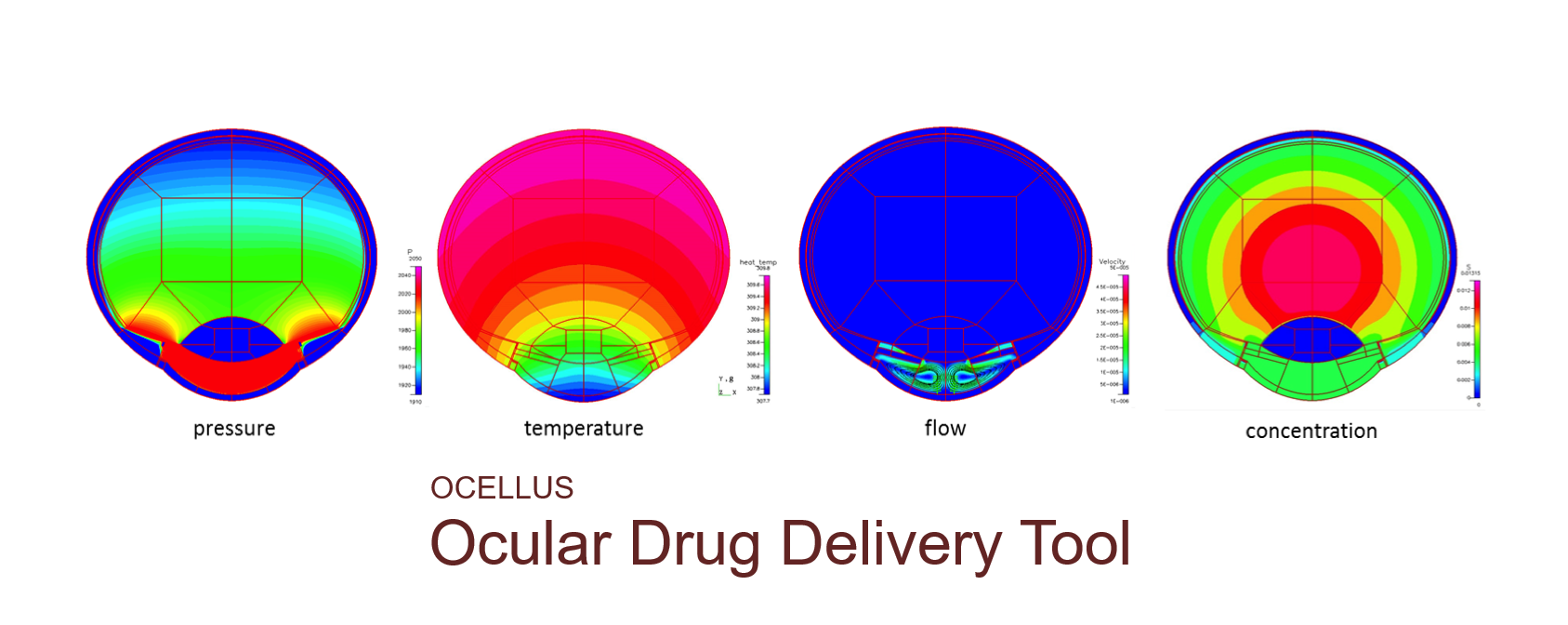
We have developed a unique multiscale-multiphysics model to simulate ocular delivery, absorption, distribution, pharmacokinetics and in vitro to in vivo extrapolation (IVIVE) for generic drugs and drug products.
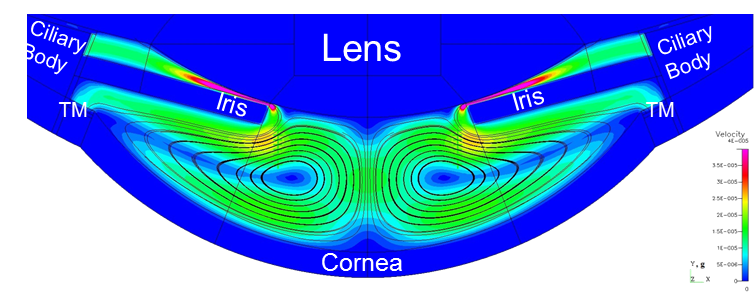
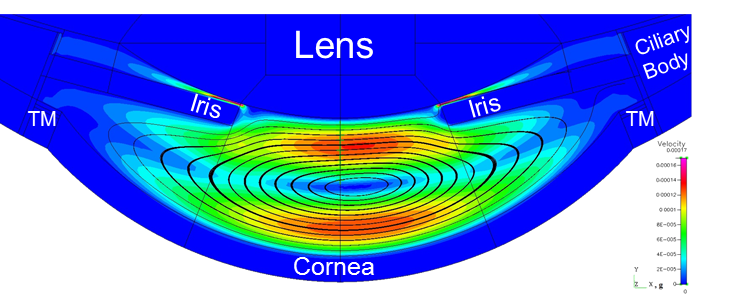
High-fidelity 2D Ocular Model
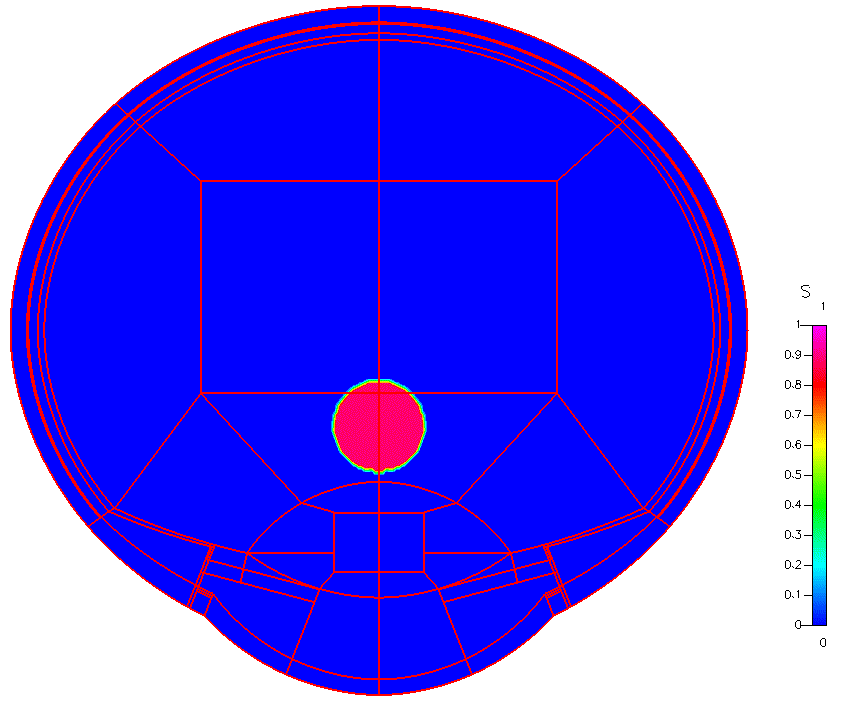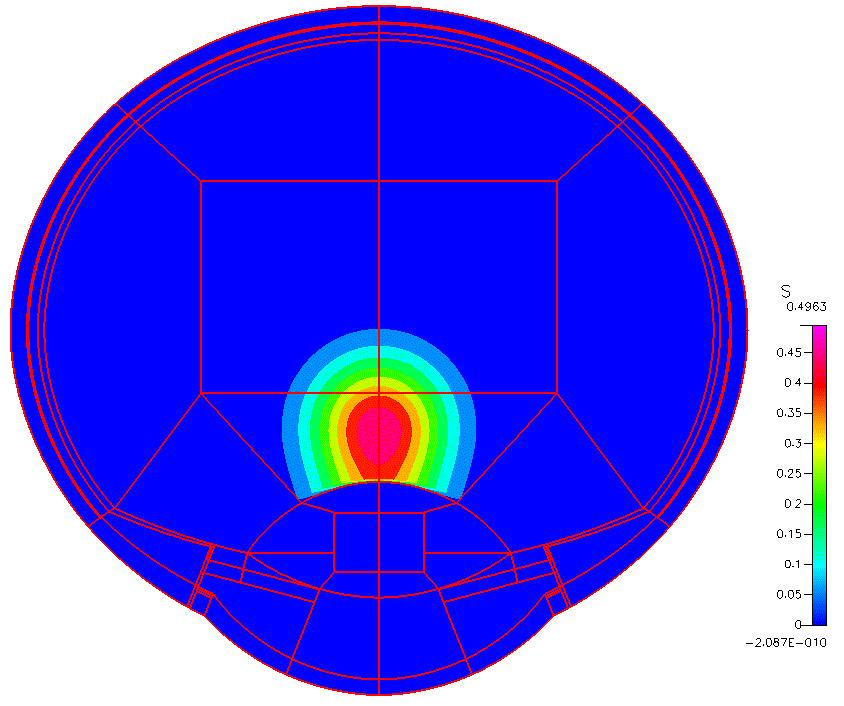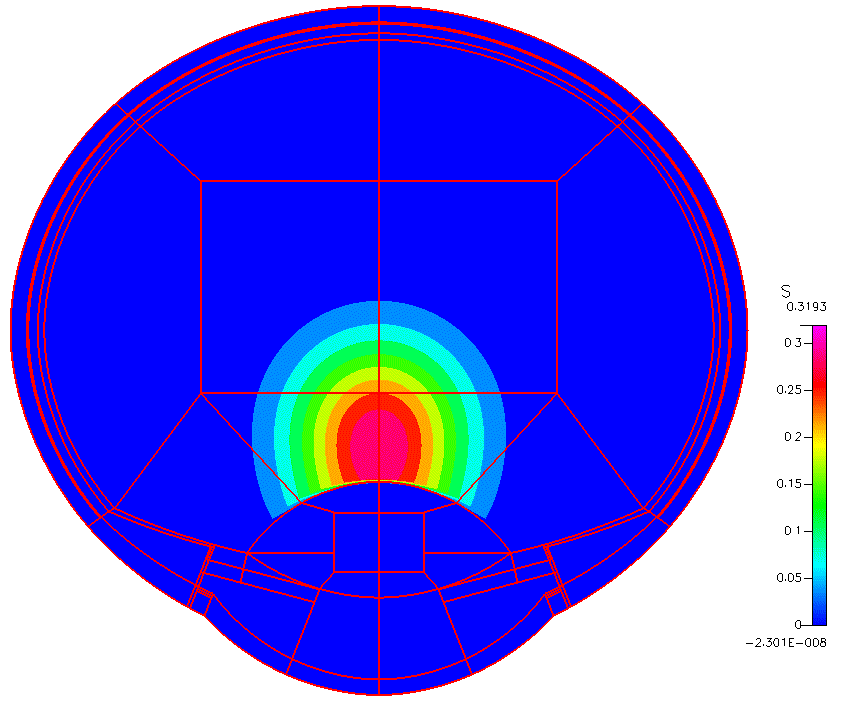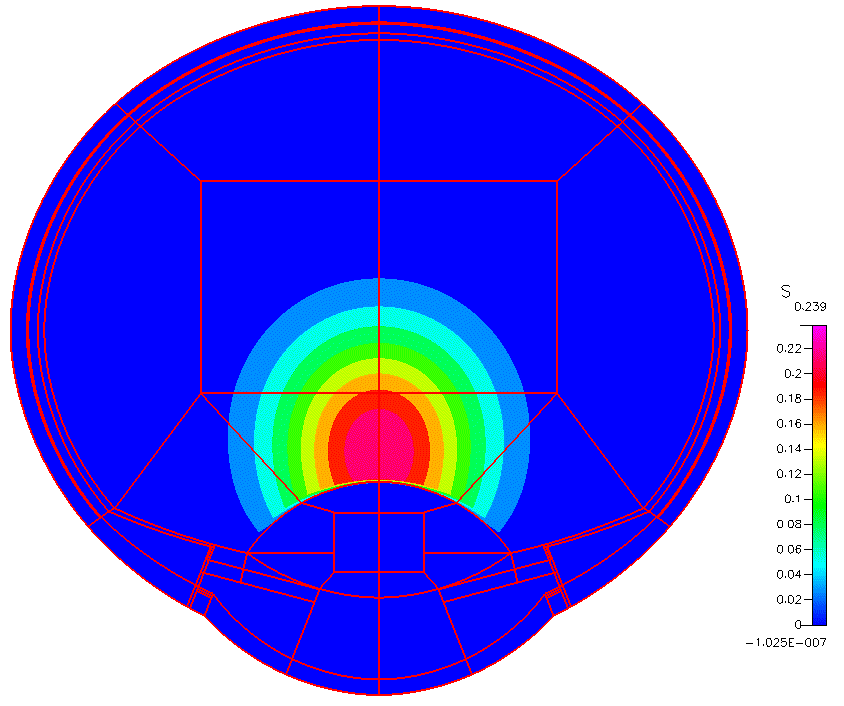
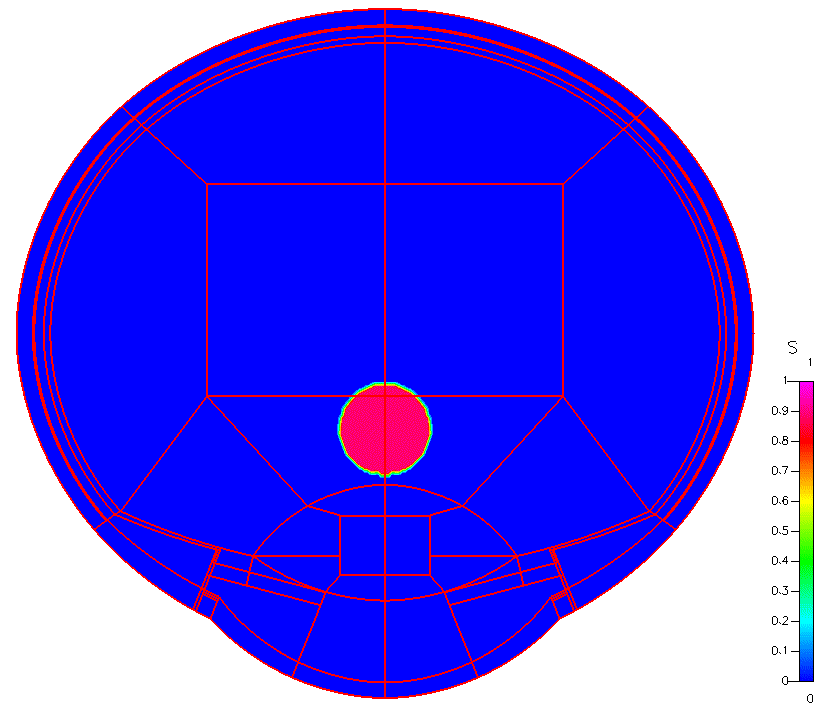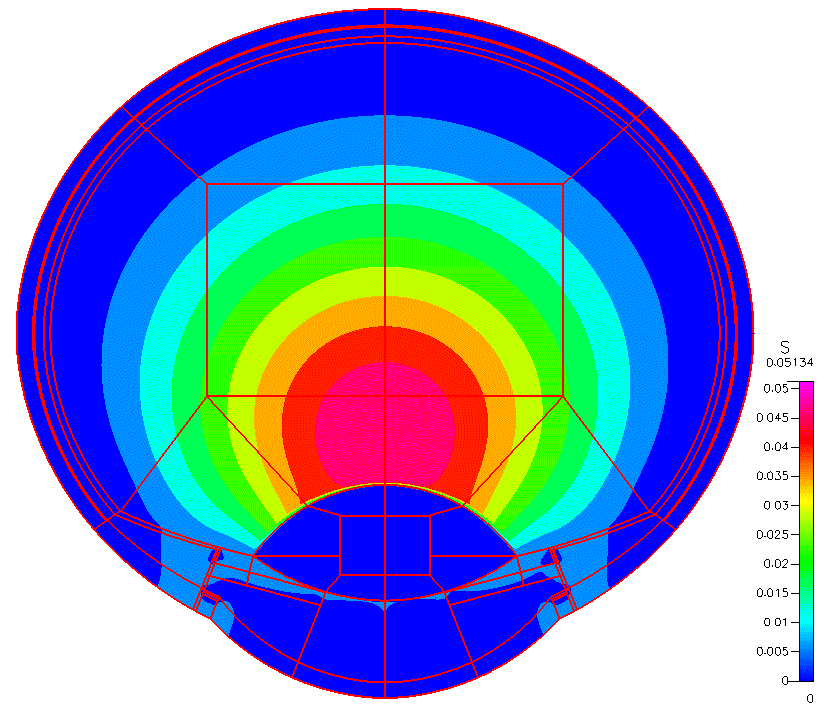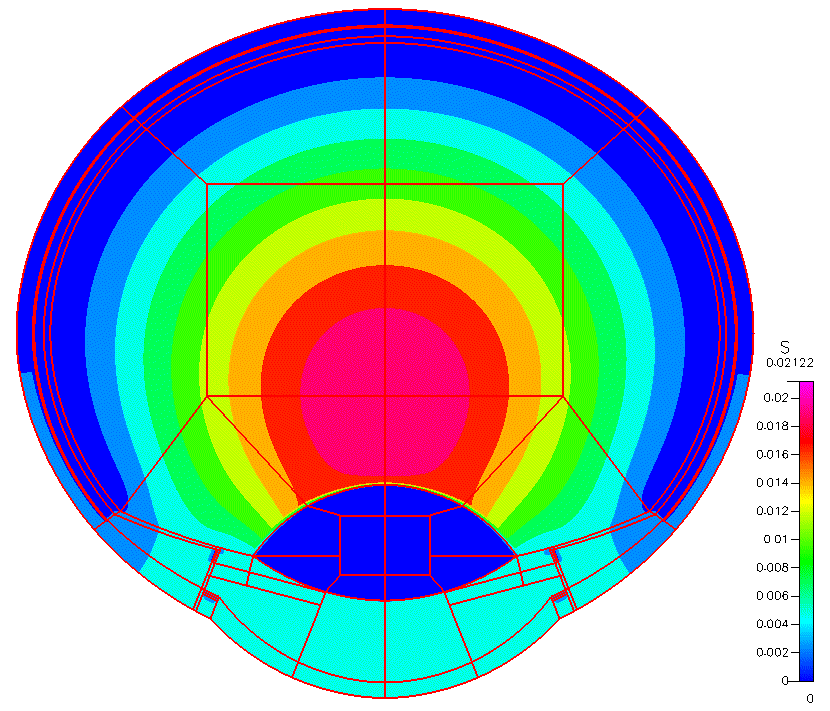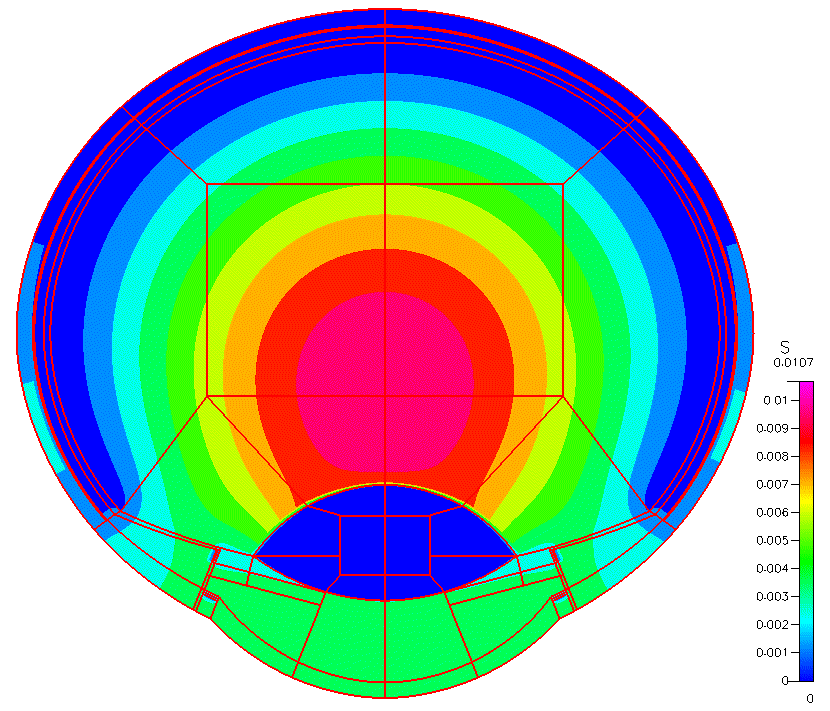
This project was funded by FDA 1U01FD005219-01.
Contact us for more information and to download a copy of Ocellus.